Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. The ground state of a quantum field theory is usually called the vacuum state or the vacuum.
If more than one ground state exists, they are said to be degenerate. Many systems have degenerate ground states. It turns out that degeneracy occurs whenever a nontrivial unitary operator commutes with the Hamiltonian of the system.
According to the third law of thermodynamics, a system at absolute zero temperature exists in its ground state; thus, its entropy is determined by the degeneracy of the ground state. Many systems, such as a perfect crystal lattice, have a unique ground state and therefore have zero entropy at absolute zero because the logarithm of 1 is 0. It is also possible for the highest excited state to have absolute zero temperature for systems that exhibit negative temperature.
Examples
- The wave function of the ground state of a particle in a one-dimensional well is a half-period sine wave which goes to zero at the two edges of the well. The energy of the particle is given by
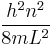 , where h is the Planck constant, m is the mass of the particle, n is the energy state (n = 1 corresponds to the ground-state energy), and L is the width of the well.
, where h is the Planck constant, m is the mass of the particle, n is the energy state (n = 1 corresponds to the ground-state energy), and L is the width of the well.
- The wave function of the ground state of a hydrogen atom is a spherically-symmetric distribution centred on the nucleus, which is largest at the center and reduces exponentially at larger distances. The electron is most likely to be found at a distance from the nucleus equal to the Bohr radius. This function is known as the 1s atomic orbital. For hydrogen (H), an electron in the ground state has energy −13.6 eV, relative to 0.0 eV when the H atom is ionized, e.g. the electron is completely removed.
| “ | the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the caesium 133 atom at rest at a temperature of 0 K. | ” |
References
- Feynman, Richard; Leighton, Robert; Sands, Matthew (1965). "see section 2-5 for energy levels, 19 for the hydrogen atom". The Feynman Lectures on Physics. 3.